In 1962 Neil Bartlett demonstrated the first reaction of a noble gas. The noble gas family of elements - helium, neon, argon, krypton, xenon, and radon - had previously been regarded as inert. By combining xenon with a platinum fluoride, Bartlett created the first noble gas compound. This reaction began the field of noble gas chemistry, which became fundamental to the scientific understanding of the chemical bond. Noble gas compounds have helped create anti-tumor agents and have been used in lasers.
Frontiers of Knowledge


When Joseph Priestley discovered oxygen in 1774, he answered age-old questions of why and how things burn. An Englishman by birth, Priestley was deeply involved in politics and religion, as well as science. When his vocal support for the American and French revolutions made remaining in his homeland dangerous, Priestley left England in 1794 and continued his work in America until his death. His library of some 1,600 volumes and his chemical laboratory, where he first isolated carbon monoxide, were probably the best in the country at that time.

In 1961, in the National Institutes of Health Headquarters (Bethesda, MD), Marshall Nirenberg and Heinrich Matthaei discovered the key to breaking the genetic code when they conducted an experiment using a synthetic RNA chain of multiple units of uracil to instruct a chain of amino acids to add phenylalanine. The uracil (poly-U) served as a messenger directing protein synthesis. This experiment demonstrated that messenger RNA transcribes genetic information from DNA, regulating the assembly of amino acids into complex proteins.
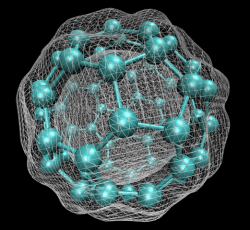
In early September 1985, a team of scientists discovered a previously unknown pure carbon molecule, C60, which they dubbed buckminsterfullerene. The name was chosen because the geodesic domes of Buckminster Fuller provided a clue that the molecule’s atoms might be arranged in the form of a hollow cage. The structure, a truncated icosahedron with 32 faces, 12 pentagonal and 20 hexagonal, has the shape of a soccer ball.

Antoine-Laurent Lavoisier studied at the Académie des Sciences de l'Institut de France (then "Collège Mazarin") from 1754 to 1761. He was elected to the Royal Academy of Sciences in 1768, where he presented his important studies on oxygen in chemistry. These began with a "pli cacheté" of Nov. 2, 1772, and, after he experimentally proved the chemical composition of water by the quantitative method, culminated in his abandoning of the phlogistic theory in 1785.

In his laboratory at Western Reserve University (Now Case Western Reserve University), Edward W. Morley carried out his research on the atomic weight of oxygen that provided a new standard to the science of chemistry. The accuracy of his analyses has never been superseded by chemical means. His great work, published in 1895, also gave important insight into the atomic theory of matter.
He observed, after carefully analysis of the volume proportions in which hydrogen and oxygen unite, that the atomic weight of oxygen was 15.879.
The plaque commemorating the event reads:
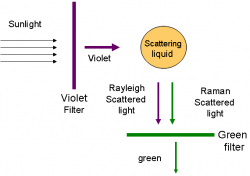
"I propose this evening to speak to you on a new kind of radiation or light emission from atoms and molecules." With these prophetic words, Professor C. V. Raman of Calcutta University began his lecture to the South Indian Science Association in Bangalore on March 16, 1928. Raman proceeded to describe a discovery that resulted from a deceptively simple experiment. Conducted far from the great centers of scientific research in the Western world, the results would capture the attention of scientists around the world and bring many accolades, including the Nobel Prize, to their discoverer.


